The Phase I trial of the Covid19 vaccine AZD1222 (also known as
ChAd0x1nCoV-19) by Oxford-AstraZeneca is now complete. The results on
the safety of AZD1222 can be found at the Lancet https://marlin-prod.literatumonline.com/pb-assets/Lancet/pdfs/S0140673620316044.pdf
I have read the paper and two things have bothered me.
The first is the number and severity of the side effects of the AZD1222 vaccine. In the phase I/II trial the AZD1222 vaccine was compared to an approved Meningitis vaccine, MenACWY. I have reproduced their Figure 2 and simplified it somewhat to make the comparison better.
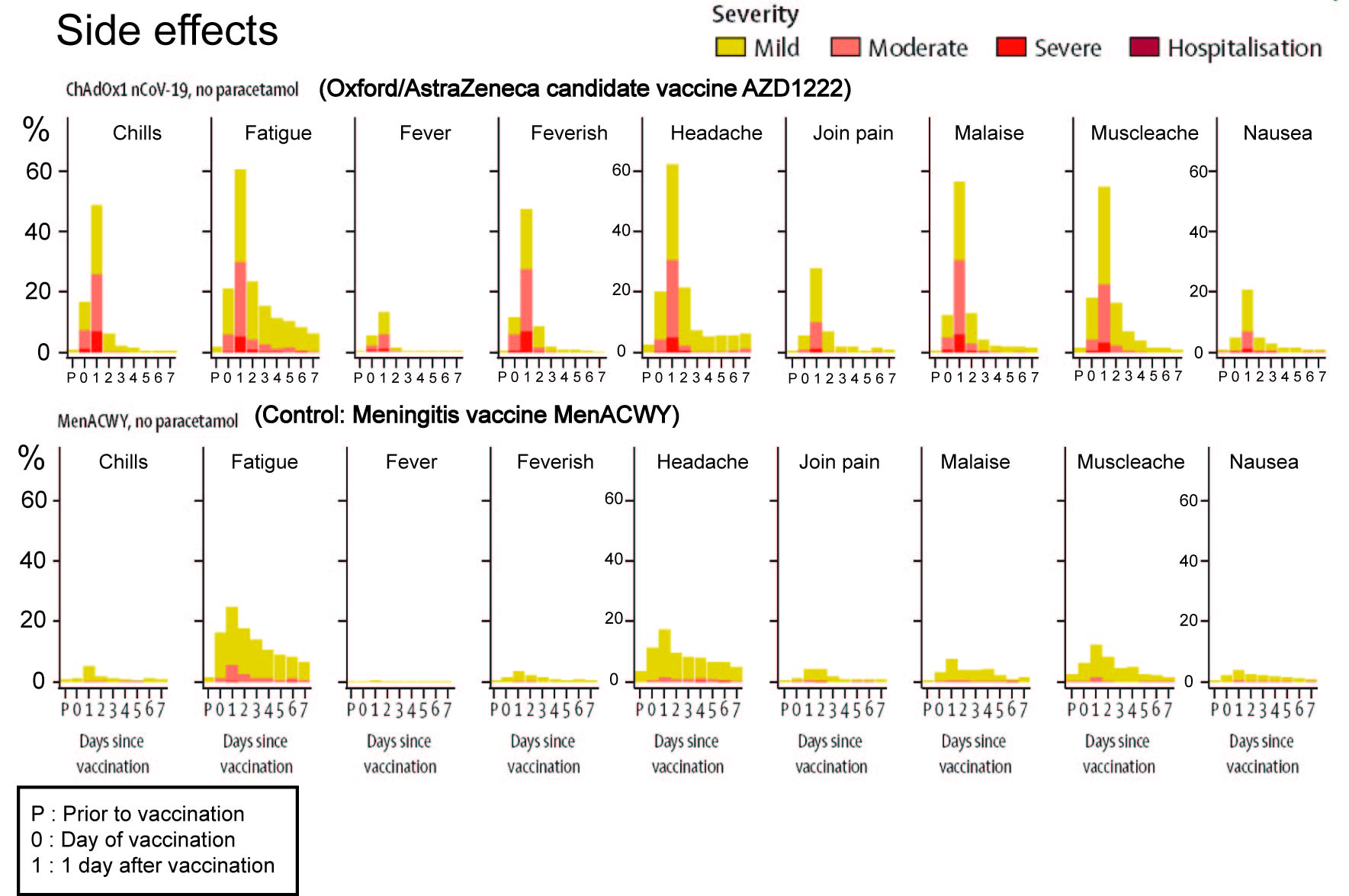
As we can see from the figure, the approved vaccine, MenACWY has no
side effects that are rated severe. It has very few side effects that
are of moderate intensity (predominantly Fatigue), and only a minority
of participants experience those. Less than 20% of participants
experience any side effect. In contrast, AZD1222 has multiple side
effects that are rated moderate to severe in intensity. Over 60% of
participants experience some form of side effects. In a word, this
vaccine is rough and this could become problematic when vaccinating
children, pregnant women and seniors. The research group attempted to
use paracetamol to ameliorate the side effects. While there were
improvements, it did not stop participants from reporting side effects
of severe intensity.
The next problem I have with AZD1222 is the week immune response elicited by the vaccine. We can see hint of this in their Figure 3, which is an ELISA experiment to gauge the strength of IgG response to viral spike protein, ie how much antibody is made that can bind to the surface proteins of SARS-Cov2 virus
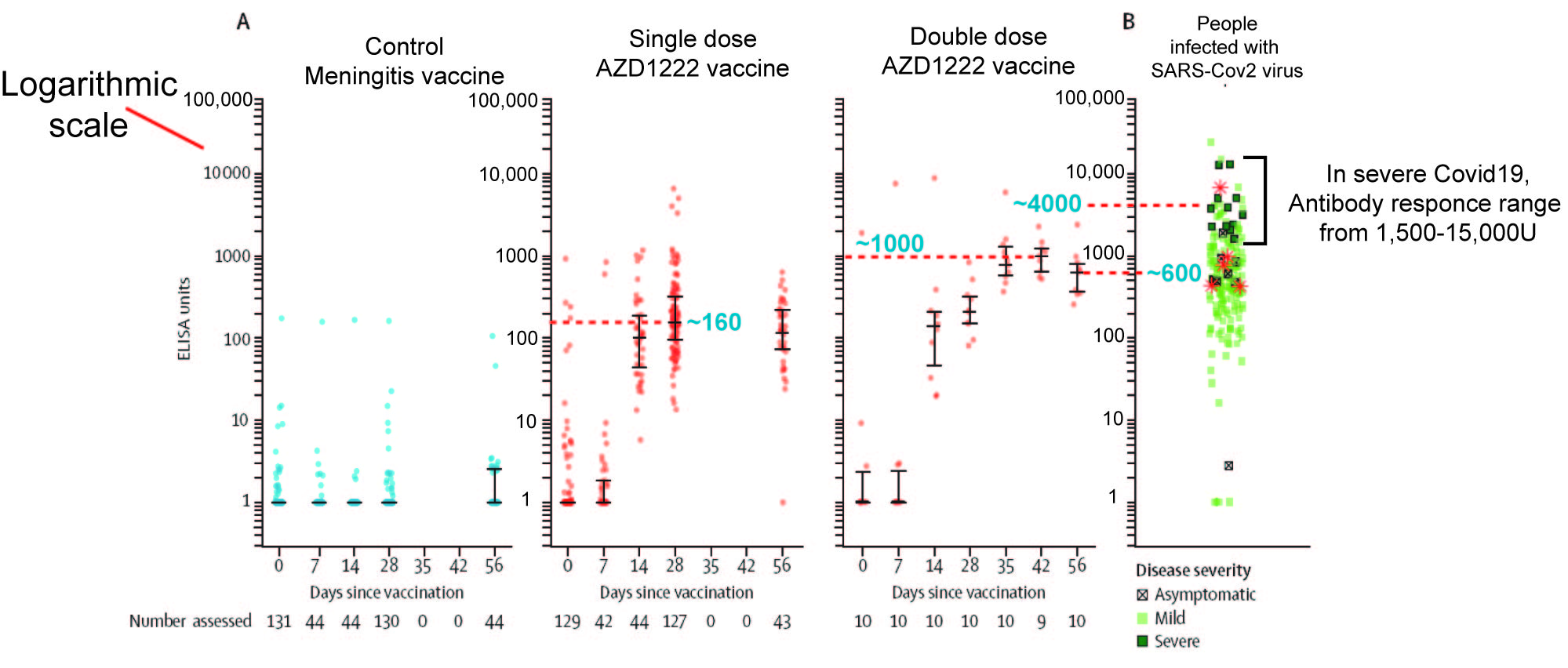 Firstly, note the y-axis of the Figure is logarithmic. A log scale
allows you to put both very big and very small numbers on the same
graph. However it also means that small differences along the axis
equate to very large differences in value. In effect, the antibody
response data is compressed, so large difference don't look so obviously
different.
Firstly, note the y-axis of the Figure is logarithmic. A log scale
allows you to put both very big and very small numbers on the same
graph. However it also means that small differences along the axis
equate to very large differences in value. In effect, the antibody
response data is compressed, so large difference don't look so obviously
different.
I have attempted to estimate the approximate mean value of important data points within the figure. As the figure indicates, this Phase I trial studied two protocols, a single dose of vaccine AZD on day 0 and a two dose protocol with one dose on day 0 and a second dose on day 28.
The average antibody value of people fighting severe Covid19 ranges from 1500 to 15,000 and has an average of ~4000 (My best guestimate from the figure).
The highest antibody value from the single dose is approximate 160 (Day 28) and has begun to fall by Day 56. This is 25 fold lower than what is generated when the body is fighting Covid19. The highest antibody value for the double vaccine dose is approximate 1000 on day 42, two weeks after the second dose. Two more weeks later (Day56) the antibody value has fallen by nearly half (~600). The highest antibody value for double dose is 4 fold lower.
This weak immune response is repeated in other experiments within the paper. Hence it makes one worry about the efficacy of the single and double dose of AZD1222 to protect against Covid19, especially in view of the rather rosy picture painted by the news media. The marketing team of AstraZeneca has gone so far as to say that they COULD be providing the UK with the vaccine as soon as September and the US by October. This is a mere 2 month from full scale production. (An impossibility unless the UK and US intend to use an untested vaccine or there is a slide of hand somewhere and it is September 2021 that they speak off.)
The optimism by the new media is in striking contrast to the trials that lie ahead for the AZD1222 vaccine. Despite the severe side effects, nobody was killed taking this vaccine, hence AZD1222 has passed the phase I trial and is considered safe enough to proceed to larger trials. It MUST be noted that passing the phase I trial does not prove that the vaccine works.
That is what a phase II/III trial is for, to answer the question that we all want to know, does this vaccine work? And if not, would a triple vaccine dose be sufficient to boast the immune system to a level high enough to take on the SARS-Cov2 virus? The AZD1222 Phase II/III trial is scheduled to be completed on August 1, 2021, just over a year from now.
I have read the paper and two things have bothered me.
The first is the number and severity of the side effects of the AZD1222 vaccine. In the phase I/II trial the AZD1222 vaccine was compared to an approved Meningitis vaccine, MenACWY. I have reproduced their Figure 2 and simplified it somewhat to make the comparison better.

The next problem I have with AZD1222 is the week immune response elicited by the vaccine. We can see hint of this in their Figure 3, which is an ELISA experiment to gauge the strength of IgG response to viral spike protein, ie how much antibody is made that can bind to the surface proteins of SARS-Cov2 virus

I have attempted to estimate the approximate mean value of important data points within the figure. As the figure indicates, this Phase I trial studied two protocols, a single dose of vaccine AZD on day 0 and a two dose protocol with one dose on day 0 and a second dose on day 28.
The average antibody value of people fighting severe Covid19 ranges from 1500 to 15,000 and has an average of ~4000 (My best guestimate from the figure).
The highest antibody value from the single dose is approximate 160 (Day 28) and has begun to fall by Day 56. This is 25 fold lower than what is generated when the body is fighting Covid19. The highest antibody value for the double vaccine dose is approximate 1000 on day 42, two weeks after the second dose. Two more weeks later (Day56) the antibody value has fallen by nearly half (~600). The highest antibody value for double dose is 4 fold lower.
This weak immune response is repeated in other experiments within the paper. Hence it makes one worry about the efficacy of the single and double dose of AZD1222 to protect against Covid19, especially in view of the rather rosy picture painted by the news media. The marketing team of AstraZeneca has gone so far as to say that they COULD be providing the UK with the vaccine as soon as September and the US by October. This is a mere 2 month from full scale production. (An impossibility unless the UK and US intend to use an untested vaccine or there is a slide of hand somewhere and it is September 2021 that they speak off.)
The optimism by the new media is in striking contrast to the trials that lie ahead for the AZD1222 vaccine. Despite the severe side effects, nobody was killed taking this vaccine, hence AZD1222 has passed the phase I trial and is considered safe enough to proceed to larger trials. It MUST be noted that passing the phase I trial does not prove that the vaccine works.
That is what a phase II/III trial is for, to answer the question that we all want to know, does this vaccine work? And if not, would a triple vaccine dose be sufficient to boast the immune system to a level high enough to take on the SARS-Cov2 virus? The AZD1222 Phase II/III trial is scheduled to be completed on August 1, 2021, just over a year from now.
